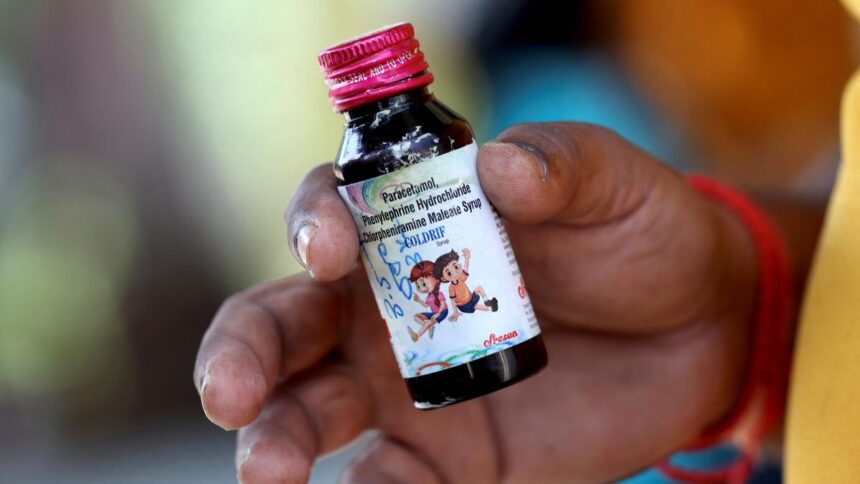
File picture of a bottle of Colddrif Cough Syrup | Photograph credit score: Reuters
The World Well being Group (WHO) is looking for clarification from India on whether or not cough syrup linked to greater than 15 little one deaths within the nation is being exported to different nations, a senior official on the international well being group mentioned on Wednesday (October 8, 2025).
The WHO has not but issued a world medical product alert for ColdRif Syrup, the cough syrup that has been blamed for the deaths of kids in Madhya Pradesh and Rajasthan. The official added that the necessity for an alert might be regarded into solely after receiving a response from the Indian well being authorities.
To this point, no less than 17 kids below the age of 5 have died in India. That is after consuming cough syrup containing the poisonous compound diethylene glycol (deg). Coldrif was manufactured by Sresan Prescribed drugs primarily based in Tamil Nadu. The corporate is at the moment investigating.
Exports of cough syrup from India are required to clear extra government-mandated laboratory exams since 2023 after greater than 140 kids died in Gambia, Uzbekistan and Cameroon. The deaths have been linked to syrup made in India.
The central authorities, in an order dated December 18, 2023, had mentioned that fixed-dose mixture (FDC) of Chlorpheniramine Maleate IP 2mg and Phenylephrine HCL IP 5mg drops/ml “shouldn’t be utilized in kids under 4 years of age”. Prescribed to deal with chilly and cough signs corresponding to a chilly nostril, sneezing, sore throat, and watery eyes, Coldrif accommodates chlorpheniramine maleate, paracetamol, and phenylephrine.
The order issued by the Central Pharmaceutical Requirements Management Group and signed by Rajeev Singh Raghuvanshi, Drug Controller Common of India, has ordered “assertion of warnings” on labels and bundle inserts on this regard.
Commerce group advisory
The All India Group of Chemists (AIOCD) on Wednesday (October 8, 2025) wrote to drug producers and advertising and marketing corporations on the directives issued by the Ministry of Well being and Household Welfare (MOHFW) and the Central Drug Normal Management Group (CDSCO).
“We’ve got noticed that in some inventories in the marketplace, this data is lacking or not prominently displayed, which may result in violations and potential misuse. Points straight associated to affected person security and regulatory compliance immediate all manufacturing and advertising and marketing authority holders to right away assessment and be certain that all merchandise adjust to these statutory labeling norms.
It urged asset personnel, distributors and area personnel to advise and information accordingly, thereby guaranteeing correct communication and compliance is maintained all through the availability chain, in full compliance with authorities notifications and circulars.